The human memory is akin to a storehouse where all knowledge is stored. Humans use this knowledge to perform a variety of tasks. One downside of human memories is that they are not accurate and authentic. This happens because information stored in memory is a gist of the true events that happened in real time which is not the exact copy of the event itself. This happens because previously-stored knowledge representations in memory influence the storage of new knowledge (Bartlett, 1932). This is one primary reason why facts retrieved from memory vary vastly from facts initially stored in memory (Loftus, Feldman, & Dashiell, 1995). A good demonstration of this effect is the phenomenon of false memories where subjects claim to remember events and incidents they have never encountered in reality. False memories show high semantic association with real events previously encoded in memory (Roediger & McDermott, 1995; Toglia, 1999) where subjects by reporting their false memories show high confidence upon the accuracy of false memories. False memories are quite similar to true memories as they are thought to undergo similar processes of encoding - where knowledge about events is learned, consolidation – where memory traces are reorganized and stabilized, and retrieval - the process which helps in accessing stored knowledge about events. One aspect in which false memories deviate from true memories is authenticity.
Previous research suggests that sleep plays a decisive role in memory formation (Born, Rasch, & Gais, 2006; Rasch & Born, 2013; Stickgold, 2005). Sleep benefits memory consolidation through offline processing of previously-stored representations (Diekelmann & Born, 2007). Neurobiological processes during sleep help in combining new memory traces with appropriate neural circuits that previously exist. These new memory traces get associated with neural circuits distributed over various brain regions. The new memory traces start integrating with pre-existing memory circuits embedded within the various brain regions. Thus, sleep is believed to promote both synaptic and system consolidation (Diekelmann & Born, 2007; Dudai, 2004). The neurobiological process of sleep-dependent memory consolidation integrates and reorganizes fresh memory traces to other pre-existing memory circuits (Dudai, 2004; Fischer, Nitschke, Melchert, Erdmann, & Born, 2005). Because of this, memories that bear very little resemblance to memories learned at encoding are generated (Fenn, Nusbaum, & Margoliash, 2003). The newly-formed memories are considered false as they are projections of actually encoded knowledge representations and are produced by generalizing the semantic relations of the original representations. Thus, the neurobiological processes that are part of sleep dependent memory consolidation result in the formation of false memories.
Another possible reason for the formation of false memories is disturbances that occur during retrieval of memory representation. Stress and impaired cognitive functioning during memory retrieval produce active interferences that may lead to incorrect source of reality monitoring of memory representations (Durmer & Dinges, 2005; Harrison & Horne; 2000). This in turn leads to the generation of false memories. The deprivation of sleep can also lead to the formation of false memories. Prolonged deprivation of sleep often results in impaired cognitive functions like vigilance, attention, and working memory and thinking (Durmer & Dinges, 2005). The reduction in vigilance, attentional span and working memory caused by the deprivation of sleep can interfere with the accurate retrieval of memory representations leading in turn to the rise of false memories.
The hypothesis whether deprivation of one night of sleep could increase formation of false memories was tested in the study. This prediction is made since deprivation of sleep leads to the decrement in several higher cognitive functions. These decrements will eventually lead to the decreased source monitoring and accurate retrieval of memory leading to increased false memory. Previous studies on sleep deprivation and false memory (Diekelmann, Landolt, Lahl, Born, & Wagner, 2008; Fenn, Gallo, Margoliash, Roediger, & Nusbaum, 2009; Rasch & Born, 2013; Stickgold, 2005) were inconclusive in their results, so it would be important to test this relationship. The Deese, Roediger, McDermott (DRM) false memory paradigm (Deese, 1959; Roediger & McDermott, 1995) was utilized for testing false memory across nights filled with sleep and sleep deprivation. The present study utilized a within-subject design where each subject participated under both the sleep filled and sleep deprived experimental nights. Thus, each participant was part of both the sleep and sleep deprivation group. The DRM requires volunteers to learn list of words (e.g., ‘bed’, ‘night’, ‘quilt’….) which are highly related semantically. The word with the highest semantic relation with the list words - the critical lure (‘sleep’) (defines the category that list words belong) is absent from the list. Later volunteers are asked to retrieve back the list words and it is reported that at retrieval volunteers falsely report the critical lure as the list word with very high probabilities (Roediger & McDermott, 1995). In the present study, a variation of the DRM was employed where at retrieval a list containing words from learned list, the critical lure, unrelated words to the list and weakly related words to the list words was presented to the subject. The subjects were required to make ‘old’ or ‘new’ recognition for the list words presented to them during retrieval.
Method [TOP]
Participants [TOP]
Forty healthy undergraduate males (age: 20.15 ± 2.01 (M ± SD), range 18 – 22 years) from the Indian Institute of Technology Guwahati, participated in the study. Volunteers for the study responded to flyers detailing the requirements of participation for the study. Volunteers of the study were screened for serious psychological and medical conditions. All subjects reported regular sleep-wake cycle (≥ 7 h sleep per night) with no acute variation in nighttime sleep for one month prior to the experimental nights. Prior to the experiment, subjects were instructed to refrain from alcohol and caffeine for the entire duration on the study, starting one day prior to the first experimental night. The present study used Actiwatch (Philips Respironics, 2013) to monitor subjects sleep related activities. Subjects spent an adaptation night with no learning and Actiwatch at their residences prior to the start of the experiment to establish baseline data. Subjects on the experimental sleep night slept 08 hours sleeping at their residence with Actiwatch and thirty-six hours later after a recovery night sleep, returned for retrieval testing. On the deprivation night, the subjects stayed back with the experimenter for the entire night in the sleep laboratory after which they returned to the laboratory for retrieval testing following a recovery night sleep. Subjects were allowed to eat light snacks, drink water, watch movies, complete academic tasks or take brief walks to keep them awake during the deprivation night. All subjects provided informed consent for the experimental protocol. Subjects received monetary compensation for their participation. The local human ethics committee at IIT Guwahati approved the study.
Design and Procedure [TOP]
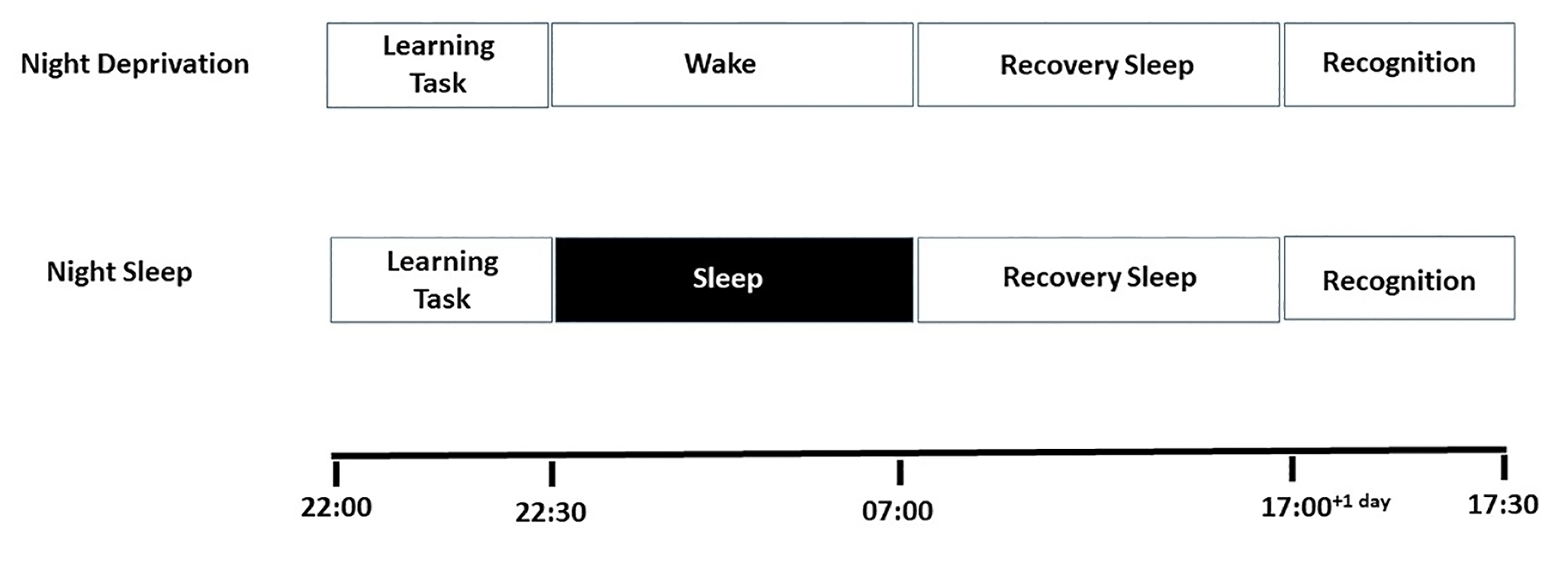
The present study used a complete repeated measure design under which each subject took part on both the experimental nights (sleep and deprivation) (see Figure 1). The order of participation for the nights was randomized. On the ‘night sleep’ experimental night, subjects performed the list learning at 21:00h. After learning, subjects received the Actiwatch with instructions for not removing the Actiwatch at any point during the night. Additionally, subjects received instruction for returning to the laboratory for retrieval testing thirty-six hour later (following an additional recovery night sleep). Subjects then returned to their residence where they slept from 22:00h to 07:00h. Upon their return to the laboratory, subjects’ Actiwatch data was downloaded and stored offline and the subjects completed retrieval testing. On the ‘night deprivation’ experimental night, subjects performed the list learning at 21:30h. Subjects after finishing the learning task remained awake in the sleep laboratory with the experimenter from 22:00h to 07:00h the next morning and left the laboratory at 07:30h. Following a recovery night sleep subjects returned at 17:00h for retrieval testing. Subjects on both the experimental nights performed on a word retention test prior to retrieval testing as a part of control testing. The present design aimed at explaining how the deprivation of sleep modulated false memory formation. This aim was intended to be achieved by comparing the difference in performance at retrieval between the night deprivation and night sleep conditions.
Figure 1
Experimental Design, subjects either slept or awaked on the experimental nights after the learning session. Following a recovery night sleep subjects performed on recognition testing thirty-six hours after experimental night
False Memory Task [TOP]
The present study used the standard Deese, Roediger, and McDermott (DRM) (Roediger & McDermott, 1995) task to study false memory formation. Subjects in the study learned 10 DRM lists with each list having 12 highly sematic associated words selected from Stadler, Roediger, & McDermott (1999). The critical lure word that has the highest sematic association with the list words was not included in the list. Each list had words arranged in descending order of sematic relation with the lure word. This means that the words with the strongest association were at the top of the list and the ones with the weakest association were at the bottom of the list. The words were presented using E-Prime software (“Psychology Software Tools”, n.d.) where each list word stayed on the screen for one second and the inter-stimulus interval was one second. The gap of ten-second separated successive presentations of two lists. The lists were displayed using HP EliteDispaly (E201) 21-inch LED monitor (1920 x 1360 pixel) run on Intel powered computer running Windows. A visual angle of 2 degree was maintained between the display and subjects’ eyes (McCready, 1985) and the distance of the monitor from the subjects’ eye level was one foot. Subjects were advised to direct complete attention to the presented words and memorize them for a test later.
The retrieval testing for word recognition was completed using E-Prime software. The retrieval test consisted of three classes of words: old list words (20 words from the list presented at learning), distractor words (20 words that were unrelated to the words presented at learning) and critical lures (10 words with the highest sematic association with the list words). Each word appeared on screen for one second after the which the subject was required to make old/new judgment and record their response using the keyboard key combinations (x = old; m = new). The next words in the sequence appeared only after subjects successfully registered the desired responses.
Two versions of the DRM lists and recognition test were prepared for testing subjects on the sleep and sleep deprivation nights. Subjects were randomly assigned to the sleep / deprivation experimental nights and the order of presentation of the test stimuli was randomized across subjects.
False memory rates were calculated for the sleep and sleep deprivation experimental nights. On sleep nights subjects performed the learning task at 21:00 hours and then slept for 8 hours with sleep monitoring using Actiwatch (Philips Respironics, 2013) (see Table 1). During sleep deprivation nights after learning at 21:00, subjects stayed back in the laboratory under the supervision of the experimenter where they remained awake for the entire night. Subjects under both the nights completed retrieval task thirty-six hours later after a recovery night sleep following experimental nights.
Control Variables [TOP]
At the beginning of both the learning and retrieval sessions subject had to complete a list of tests that measured their concentration and motivation. The scales were designed in-house and followed a five-point Likert scale paradigm with one meaning not at all and five meaning completely true (see Table 2). Their reliability and validity were assessed on a sample of 300 students for a different study in the past. These scales were part of inclusion/exclusion battery used by the authors in several experiments. Mental concentration scale has items that measure concentration (e.g., I have difficulty concentrating on task for more than 5 min, I am easily distracted by background noises, etc.). Chronbach’ alpha for this scale > .7 and the concurrent validity of this scale with the MMSE (Folstein, Folstein, & McHugh, 1975) was significant. Motivation scale had items borrowed from the Intrinsic motivation inventory (Deci & Ryan, 1985; Monteiro, Mata, & Peixoto, 2015). Some items are (I put a lot of effort into doing the experiment, I enjoyed doing the experiment, etc.). This scale also had Chronbach’ alpha > .7 and the concurrent validity of this scale with the original Intrinsic Motivation Inventory was significant. Stanford sleepiness scale (SSE) was used to measure the participants’ subjective sleepiness (Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973).
Table 2
Recognition Memory Performance
| Session / Measure | Night Deprivation | Night Sleep |
|---|---|---|
| Learning | ||
| Motivation | 3.01 ± 0.09 | 2.95 ± 0.06 |
| Concentration | 3.21 ± 0.11 | 2.89 ± 0.10 |
| Stanford Sleepiness Scale | 2.68 ± 0.02 | 2.54 ± 0.10 |
| Retrieval | ||
| Motivation | 3.02 ± 0.12 | 3.18 ± 0.10 |
| Concentration | 3.00 ± 0.08 | 3.13 ± 0.11 |
| Stanford Sleepiness Scale | 2.36 ± 0.11 | 2.25 ± 0.14 |
Statistical Analysis [TOP]
Subject false memory data collected from the study was scanned for outliers and the remaining data was made available for analysis. Data from one subject had to be rejected on grounds of technicality and the remaining data from 39 subjects were utilized for data analysis. Data analysis utilized the standard procedure for measuring recognition memory where hit and false alarm rates of false memories both acted as dependent variables of interest. In addition, the discrimination and Bias indexes namely, Pr and Br were also computed for hits and false memories following the recommendations of the two-threshold model of recognition memory (correct recognition: Pr = hit rate – false alarm rate; false recognition: Pr = false memory rate – false alarm rate; Br = false alarm rate / (1 – Pr) for correct and false recognition) (Snodgrass, & Corwin, 1988). The recognition measures were compared between groups using paired sample t-test.
Results [TOP]
Control Variables [TOP]
Subjects were made to perform on tests of mental concentration, motivation and Stanford sleepiness scale both before the learning and retrieval sessions. Data obtained from the scales were statistically analyzed using paired sample t-test. It was reported that subjects did not significantly differ across learning and retrieval on the mental concentration test [t(38) = 1.29, p = .205], motivation test [t(38) = 1.83, p = .075], and Stanford sleepiness scales [t(38) = 1.79, p = .081].
False memory [TOP]
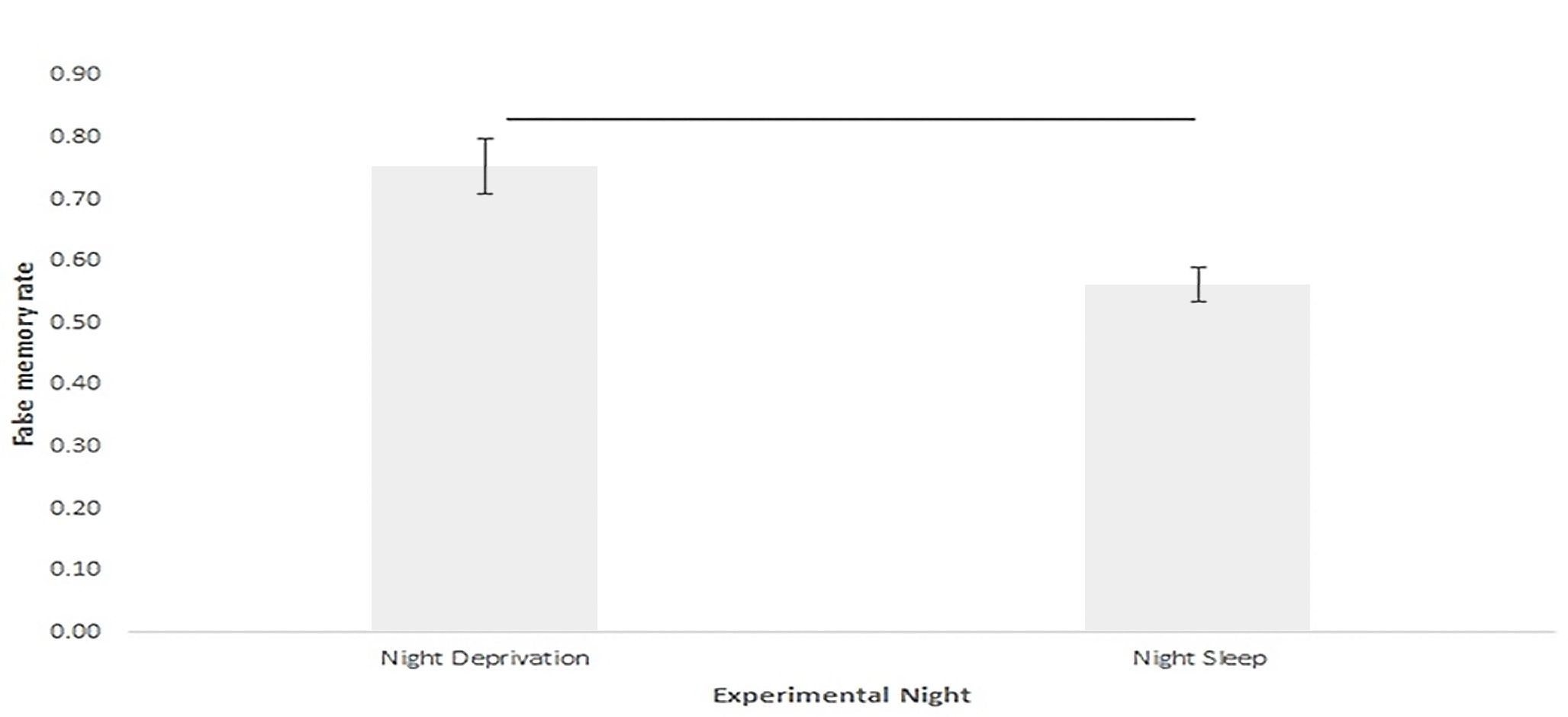
One primary result of the present experiment was that subjects after sleep deprivation reported more false memories at retrieval [t(38) = 7.27, p < .001 for pair-wise comparison] (see Figure 2). Subjects performing retrieval after sleep deprivation on an average falsely recognized 0.75 ± 0.05, i.e. 75% of the critical lures while after sleep the average false memory recognition was reported as 0.56 ± 0.03, i.e. 56% of the critical lures. Additionally, testing on the sleep and sleep deprivation night revealed that there were significant differences between hits rate (correctly recognized old words) [t(38) = 4.02, p < .001] and false alarm rate (false recognition of distractor word as old word) [t (38) = 9.02, p < .001] (see Table 3). This would further suggest that while sleep deprivation increased false memories, sleep enhanced recognition of old stimuli and decreased false alarms.
Further analysis was performed with the discrimination index P, and the response bias index B, following the two-threshold framework of recognition memory. The result of the analysis suggests that subjects on sleep deprivation nights reported more false memories as compared to on the sleep nights (Pr = 0.56 ± 0.06; 0.27 ± 0.04, for ‘sleep deprived’ and ‘sleep’ nights [t(38) = 9.34, p < .001]. Correct recognition memory also differed between groups (Pr = 0.20 ± 0.01; 0.49 ± 0.05, for ‘sleep deprived’ and ‘sleep’ nights, t(38) = 10.04, p < .001). The response bias for both the false recognition and correct recognition did not differ between the groups (false recognition: 0.44 ± 0.07, 0.42 ± 0.10, [t(38) = 1.97, p < 0.05], and correct recognition: 0.40 ± 0.07, 0.49 ± 0.13, [t(38) = 1.79, p = .081]).
Figure 2
Proportion of false memory at retrieval (recognition test), under sleep deprivation the proportion of false memory was higher than under sleep condition
These results suggest that sleep deprivation after learning leads to formation of more false memories as compared to post learning sleep. Additionally, subjects when deprived of sleep produced less correct recognition of list words from learning than when they were allowed to sleep following learning. One night of recovery sleep followed both the deprivation and sleep filled experimental nights. The recovery night ascertained that any difference in recognition memory at retrieval was because of deprivation or sleep following learning the word lists.
Discussion [TOP]
In the present paper, the effect of sleep on false memory generation for the DRM task was investigated. One aim of the present paper was to evaluate if sleep induced consolidation process led to an increment or decrement of false memory generated on DRM task. Previous work on sleep and false memory has tested immediate recognition following sleep and deprivation (Diekelmann, Landolt, Lahl, Born, & Wagner, 2008; Fenn, Gallo, Margoliash, Roediger, & Nusbaum, 2009). The results from these studies suggest a 44% increase in false memory following deprivation as compared to sleep. In the present study, long terms effects of sleep deprivation and sleep on false memory generation was a primary objective of interest. The results from the present experiment suggest that the sleep dependent consolidation process after learning leads to lesser false memory formation when tested after one night of recovery sleep. Thus, sleep deprivation following learning of the DRM list leads to enhancement of false memory production when the retrieval testing is conducted post recovery night sleep.
The increase in false memory formation under sleep deprivation can be attributed to the neurobiological states of the brain undergoing sleep deprivation. The absence of large variation in the hit rates and false alarm rates on the sleep deprivation session suggests that increased false memory rates under sleep deprivation were not the result of lower motivation caused by boredom or reduced compliance during the sleep deprivation sessions. It was found that subjects did not differ on both the sleep and the sleep deprivation sessions on the variables of motivation or compliance. Additionally, subjects under sleep deprivation followed the response criteria similar to sleep for making decisions on the ‘old-new’ recognition task. This is indicated by similar response bias for both the groups. This result suggests that high false memory produced during the sleep deprivation sessions is not the result of a liberal response criterion adopted by the subjects. From the above results, it could be concluded that the increased formation of false memories after post learning sleep deprivation is primarily linked to the altered brain state following sleep deprivation as compared to post learning sleep.
One another reason based on evidence from previous research for the increased false memories after sleep deprivation is that false memories lead to larger activation of the prefrontal cortex when compared with true memories (Schacter et al., 1996). Research evidence suggests that sleep deprivation interferes with the normal functioning of the prefrontal cortex (Drummond, Brown, Gillin, Stricker, Wong, & Buxton, 2000; Harrison & Horne, 2000; Yoo, Hu, Gujar, Jolesz, & Walker, 2007). This in turn reduces performance on cognitive tasks that rely on the prefrontal cortex (Durmer & Dinges, 2005; Harrison & Horne, 2000; Yoo, Hu, Gujar, Jolesz, & Walker, 2007). Curran, Schacter, Johnson & Spinks (2001) conducted an ERP (Event-Related Potential) study on subjects who showed higher accuracy in differentiating between old and new items and compared them with subjects reporting poor accuracy of recognition. High performers reported positive late frontal ERP as compared to low performers on the old-new recognition paradigm. One interpretation of this study can be that high performers activated more of the prefrontal cortex as compared to poor performers. The study provides direct support to our experimental results. It can be safely concluded that the disruption of the prefrontal activity through sleep deprivation leads to poorer ability for making choices between truly encountered words and internally generated words on the DRM (Yoo, Hu, Gujar, Jolesz, & Walker, 2007). This leads to generation of more false memories during sleep deprivation. Besides memory, functioning of some other cognitive functions like attention and arousal also gets affected due to disruption of the prefrontal activity (Durmer & Dinges, 2005). Knott & Dewhurst (2007) have shown a negative relationship between arousal levels at retrieval and generation of false memories on DRM task.
The results of the present study imply that false memories formation increases after sleep deprivation. However, our results do not highlight the contribution of post learning sleep dependent consolidation process on false memory generation. Results of the present study suggest a moderate increase in hit rates (correct recognitions) after sleep as compared to false memory rates. Previous research on the effect of post-learning sleep on recognition memories has indicated significant improved recognition memories across sleep as compared to deprivation (Hu, Stylos-Allan, & Walker, 2006; Wagner, Kashyap, Diekelmann, & Born, 2007). The present study reports that the sleep deprivation led to generation of more false memories while post learning sleep had no significant effect of false memory formation. Additionally, the present research suggests that the reason for the generation of false memory under sleep deprivation may be mainly due to the change in activity of the prefrontal cortex that causes the subject to inaccurately recognize the never presented items as previously seen item.
Conclusion [TOP]
The study results suggest the need for use of more sensitive procedures to test the effects of sleep on false memory in future research. In addition, the above study should be conducted with free recall procedure for getting a clear overall picture of the role of sleep in false memory formation.

 Contents
Contents This is an open access article distributed under the terms of the Creative Commons
Attribution License (
This is an open access article distributed under the terms of the Creative Commons
Attribution License (